Introduction: T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive neoplasm that accounts for 25% and 15% of adult and pediatric ALL, respectively. The prognosis is particularly poor in patients with relapsed disease, justifying the research for novel predictive markers of relapse.To date, a significant series of adult and pediatric T-ALL with analysis using high-resolution SNP-array is still missing.
Methods: Here, we report a comprehensive high-resolution SNP-array profiling in a large well-characterized cohort of 317 T-ALL patients all uniformly treated according to the French FRALLE and GRAALL protocols. SNP-array results have been correlated with clinico-biological data including age range, immunophenotype, oncogenetic and survival data.
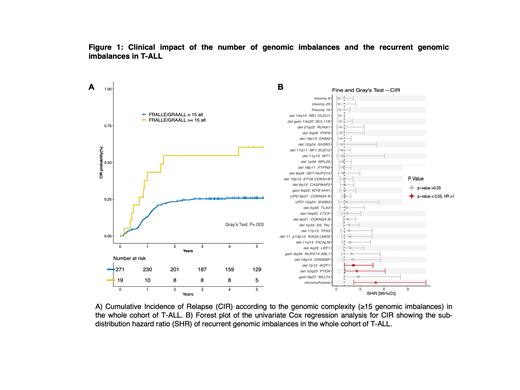
Results: The SNP-array analysis detected at least one genomic imbalance (CNVs and UPDs combined) in 304/317 (96%) T-ALL cases with a mean of 6.3 (range 0-47) genomic imbalances per sample. Among genomic imbalances detected by SNP-array analysis, we identified 35 recurrent genomic imbalances (i.e. observed in at least 2% of T-ALL patients). The most common recurrent CNV was the del(9p) including CDKN2A/B at 9p21 (~70%). Unexpectedly, the second more frequent CNV was del(13q) minimally including RB1 and/or DLEU1 at 13q14 (~14%). The third more frequent CNV was the del(6q) minimally encompassing CASP8AP2 at 6q15 (~11%). The following recurrent CNVs were: del(12p) minimally including ETV6 and/or CDKN1B at 12p13 (~9%), del(18p) minimally including PTPN2 at 18p11 (~9%), del(1p) minimally including RPL22 at 1p36 (~9%), del(17q) minimally including NF1/SUZ12 at 17q11 (~8%). Two regions were identified as being recurrently affected by UPDs: 9p21 ( CDKN2A/B) (~28%), and 12q14 ( SH2B3) (~2%). Chromothripsis was detected in 6 (~2%) cases. The present study revealed relative minor differences in the genomic landscape of T-ALL according to age range consisting primarily of higher incidence of del(1p36)/ RPL22, del(13q14)/ RB1/DLEU1 del(12p13)/ ETV6/CDKN1B and del(18p11)/ PTPN2 and lower incidence of del(9p21) and UPD(9p21)/ CDKN2A/B in T-ALL older than 30 years. Comparison of SNP-array results according to immunophenotype and oncogenetic mainly highlighted the specific cytogenetic pattern of the SIL-TAL1 subgroup which exhibited a low genomic complexity with a lower number of genomic imbalances per sample and a lower diversity in genomic imbalances. Using the “survcutpoint” approach, we determined that a cutoff of 15 alterations was the most significant threshold to stratify the patients into a high- and a low-risk group of relapse according to the number of alterations detected with our SNP-array approach. Compared to patients harboring less than 15 alterations (n=296), patients with ≥ 15 genomic imbalances (n=21) had a significantly shorter event-free survival (EFS) and increased cumulative incidence of relapse (CIR)(4y-EFS: 38% vs. 64%; hazard ratio (HR): 2.1, 95%CI (1.2 - 3.7); P=.008, 4y-CIR: 53% vs. 26%; subdistribution hazard ratio (SHR): 2.9, 95%CI (1.5 - 5.4); P=.001) (Figure 1A). Additionally, survival analysis revealed the poor outcome conferred by the presence of chromothripsis (n=6) as well as gain(6q27)/ MLLT4 (n=10) and del(16p13)/ CREBBP (n=15) despite the low number of affected cases (4y-EFS: 17% vs. 63%; HR: 3.1, 95%CI (1.3 - 7.8); P=.01 and 4y-CIR: 83% vs. 26%; SHR: 5.0, 95%CI (2.2 - 11.3); P <.001 for chromothripsis; 4y-EFS: 30% vs. 63%; HR: 2.5, 95%CI (1.2 - 5.4); P=.002 and 4y-CIR: 57% vs. 27%; SHR: 2.8, 95%CI (0.96 - 8.1); P=.06 for gain (6q27)/ MLLT4; 4y-EFS: 40% vs. 63%; HR: 2.5, 95%CI (1.3 - 4.8); P=.005 and CIR in the FRALLE cohort; CIR: 4y-CIR: 50% vs. 21%; SHR: 5.4, 95%CI (1.5 - 20.0); P=.001 for del(16p13)/ CREBBP) (Figure 1B).
Conclusion: Our study demonstrated that the whole genome analysis of imbalances provides new insights to refine the risk stratification in T-ALL. Notably, genomic complexity (≥ 15 genomic imbalances) and chromothripis demonstrated an association with an inferior outcome in terms of EFS and CIR in pediatric and adult T-ALL.
Disclosures
Huguet:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Clinign: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees. Dombret:Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings; Astellas: Research Funding. Baruchel:Novartis: Honoraria; Celgene: Honoraria; Servier: Honoraria; Jazz: Honoraria; Astra-Zeneca: Honoraria. Boissel:Astellas Pharma: Honoraria; ARIAD/Incyte: Honoraria; Servier: Consultancy, Honoraria, Other: Advisory role; Novartis: Consultancy, Honoraria, Other: Advisory role, Research Funding; Amgen: Consultancy, Honoraria, Other: Expert Testimony and advisory role, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal